Your Specific heat capacity of gold images are ready. Specific heat capacity of gold are a topic that is being searched for and liked by netizens today. You can Get the Specific heat capacity of gold files here. Download all free images.
If you’re searching for specific heat capacity of gold pictures information related to the specific heat capacity of gold interest, you have pay a visit to the ideal site. Our site frequently gives you hints for viewing the maximum quality video and image content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
Specific Heat Capacity Of Gold. A 4091 Wm 2 x 30 m 2 122727. Latent Heat of Vaporization of Gold is 3344 kJmol. Specific heat capacity of Gold. Al 0903 JgC Pb 0160 JgC.
 Atoms From universe-review.ca
Atoms From universe-review.ca
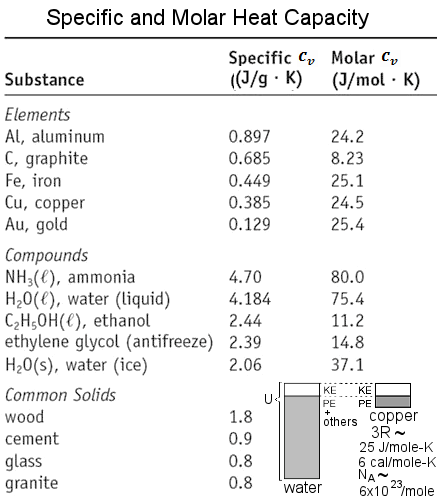
71 rows The relationship between heat and temperature change is usually expressed in the. Gold Specific Heat Latent Heat of Fusion Latent Heat of Vaporization. Determine if its endothermic or exothermic 1. Specific heat capacity in J kg C. Molar heat capacity is referring to the amount of energy required to raise 1 mole of that substance 1 degree kelvin. The specific heat of any substance is referring to the amount of energy require to raise 1 gram of that substance 1 degree kelvin.
Latent Heat of Vaporization of Gold is 3344 kJmol.
The total heat loss through this wall will be. Given that the specific heat of gold is 0129 JgºC calculate the final system temperature if a 2000 g block of gold at 1000 ºC is placed in a coffee-cup calorimeter containing 500 g of water at an initial temperature of 250 ºC. Specific heat is defined as the amount of heat required to increase the temperature of one gram of a substance by one degree Celsius. Gold has a specific heat of 0129 JgC. Where Q is the energy added and ΔT is the change in temperature. 7 rows Gold Specific Heat.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
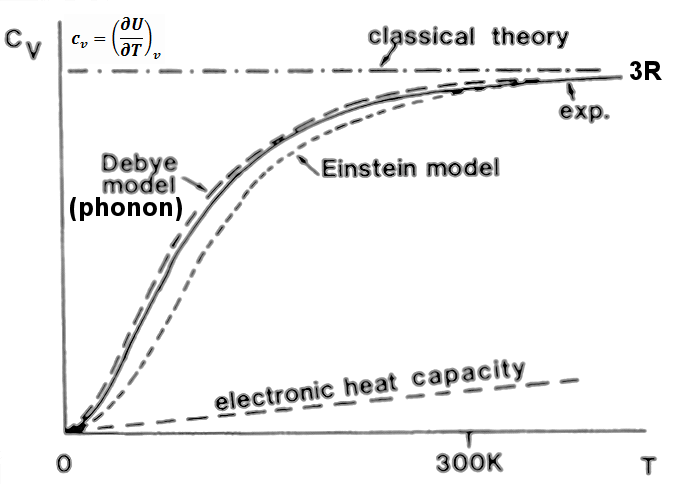
What Is the Specific Heat Capacity of Gold. Specific heat capacity in J kg C. Specific heat of Gold is 0128 Jg K. Below this table is an image version for offline viewing. The specific heat capacity during different processes such as constant volume Cv and constant pressure Cp are related to each other by the specific heat ratio ɣ CpCv or the gas constant R Cp - Cv.
 Source: pinterest.com
Source: pinterest.com
The specific heat capacity of gold is 0128 Jg ºC. Who are the experts. Experts are tested by Chegg as specialists in their subject area. Spezifische Wärmekapazität von Gold C ist 0129 kJ kgK. Specific heat capacity in J kg C.
 Source: pinterest.com
Source: pinterest.com
Determine if its endothermic or exothermic 1. Specific heat of Gold is 0128 Jg K. The formula for specific heat capacity C of a substance with mass m is C Q m ΔT. Gold Specific heat capacity of Gold. Al 0903 JgC Pb 0160 JgC.

The specific heat of gold is given at a temperature of 0 C. The total heat loss through this wall will be. So in order to solve for molar heat capacity of gold we must convert from grams of gold to moles of gold. The specific heat of any substance is referring to the amount of energy require to raise 1 gram of that substance 1 degree kelvin. The specific heat of gold is given at a temperature of 0 C.
 Source: pinterest.com
Source: pinterest.com
The specific heat of gold is given at a temperature of 0 C. 240 x 10 3 J J 10 x 240 J 400 C 250 - 1000 x C Jg 0128 x g 2500 T c m 3 Au Au m 2 q. The formula for specific heat capacity C of a substance with mass m is C Q m ΔT. Specific heat capacity in J kg C. Latent Heat of Vaporization of Gold is 3344 kJmol.
 Source: studylib.net
Source: studylib.net
Explain how they differ from each other. Oct 16 2016. Explain how they differ from each other. 71 rows The relationship between heat and temperature change is usually expressed in the. Specific heat capacity in J kg C.
 Source: guyhowto.com
Source: guyhowto.com
What is the molar heat capacity of gold. Calculate the final temperature of the metal and water mixture assuming no heat loss to the surroundings. Heat capacity is an extensive propertyit depends on the amount or mass of the sample. Specific heat is the amount of heat energy needed to raise the temperature of one unit of mass by 1 degree Celsius. Explain how they differ from each other.
 Source: chegg.com
Source: chegg.com
Specific heat of Gold is 0128 Jg K. Specific heat of Gold is 0128 Jg K. Chemistry Thermochemistry Specific Heat 1 Answer WolfeHeart May 21 2018 254 Jmol Explanation. Where Q is the energy added and ΔT is the change in temperature. Molar heat capacity is referring to the amount of energy required to raise 1 mole of that substance 1 degree kelvin.
 Source: sciencedirect.com
Source: sciencedirect.com
Explain how they differ from each other. Where Q is the energy added and ΔT is the change in temperature. U 1 110 015025 130 136 Wm 2 K. Specific heat capacity of gold C is 0129 kJ kgK. Explain how they differ from each other.
 Source: theengineeringmindset.com
Source: theengineeringmindset.com
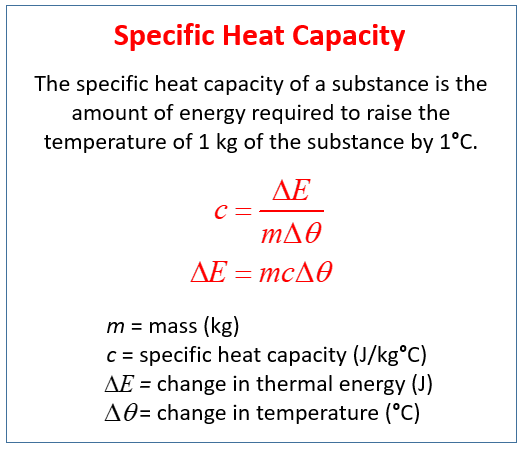
Specific heat is the amount of heat energy needed to raise the temperature of one unit of mass by 1 degree Celsius. Q m c Δ T b. So in order to solve for molar heat capacity of gold we must convert from grams of gold to moles of gold. The specific heat capacity of gold is 0128 Jg ºC. Where Q is the energy added and ΔT is the change in temperature.
 Source: researchgate.net
Source: researchgate.net
Specific heat or. If 85 JJ of heat is added to a pure gold coin with a mass of 12 gg what is the temperature change. Lawrence SawyerEGetty Images Gold at 25 degrees Celsius has a specific heat of 0129 joules per gram per degree Celsius. The specific heat capacity of materials ranging from Water to Uranium has been listed below in alphabetical order. Specific Heat of Liquid.
 Source: pinterest.com
Source: pinterest.com
Boiling Point K calg o C kJkg o C Aluminum. Specific heat of Gold is 0128 Jg K. CT abT2 and Thus in a plot of CT as a function of T2 the intercept a on the y-axis gives the value of the. By Staff WriterLast Updated March 29 2020 Follow Us. What Is the Specific Heat Capacity of Gold.
 Source: pinterest.com
Source: pinterest.com
Specific heat capacity in J kg C. Experts are tested by Chegg as specialists in their subject area. Oct 16 2016. The specific heat capacity of gold is 0128 Jg ºC. Boiling Point K calg o C kJkg o C Aluminum.
 Source: universe-review.ca
Source: universe-review.ca
Heat is a combination of kinetic energy measured by temperature and potential energy. 19697 is the molar mass of gold which can be found on the Periodic Table of ElementsMay 21 2018. Gold Specific heat capacity of Gold. The formula for specific heat capacity C of a substance with mass m is C Q m ΔT. Specific heat capacity of gold C is 0129 kJ kgK.
 Source: britannica.com
Source: britannica.com
Heat is a combination of kinetic energy measured by temperature and potential energy. Heat is not the same as temperature yet they are related. Gold Specific Heat Latent Heat of Fusion Latent Heat of Vaporization. Chemistry Thermochemistry Specific Heat 1 Answer WolfeHeart May 21 2018 254 Jmol Explanation. The specific heat capacity of materials ranging from Water to Uranium has been listed below in alphabetical order.

Specific heat is a measure of the heat capacity of a substance. Gold has a specific heat of 0129 JgC. The specific heat of gold is given at a temperature of 0 C. Specific Heat Capacity table. Q m c Δ T b.
 Source: universe-review.ca
Source: universe-review.ca
Gold has a specific heat of 0129 JgC. Total specific heat of a crystal. The specific heat of gold is given at a temperature of 0 C. A 500 g sample of aluminum specific heat capacity 089 J g 1 C 1 and a 1000 g sample of iron specific heat capacity 045 J g 1 C 1 are heated to 1000 CThe mixture of hot iron and aluminum is then dropped into 919 g of water at 237 C. Spezifische Wärmekapazität von Gold C ist 0129 kJ kgK.
 Source: guyhowto.com
Source: guyhowto.com
Experts are tested by Chegg as specialists in their subject area. Spezifische Wärmekapazität von Gold C ist 0129 kJ kgK. The specific heat capacity of gold is 0128 JgCJgC. The formula for specific heat capacity C of a substance with mass m is C Q m ΔT. Specific Heat Capacity table.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title specific heat capacity of gold by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






